

The helical conformation allows a linear arrangement of the atoms involved in the hydrogen bonds, which gives the bonds maximum strength and thus makes the helical conformation very stable. Counting from the N-terminal end, the C-O group of each amino acid residue is hydroge n bonded to the N-H group of the amino acid four residues away from it in the covalently bonded sequence. The α-helix is stabilized by hydrogen bonds parallel to the helix axis within the backbone of a single polypeptide chain. The β -pleated sheet structure can give a two-dimensional array and can involve one or more polypeptide chains. The α -helix is rodlike and involves only one polypeptide chain. Nomenclature the Pauling-Corey alpha-helix is a 3.6(13)-helix.The α -helix and β -pleated sheet are periodic structures their features repeat at regular intervals. There are main chain hydrogen bondsīetween residues separated by three residues along the chain (ie O(i) to N(i+3)). There are three residues per turn and ten atoms enclosed in a ring formed by each hydrogenīond (note the hydrogen atom is included in this count). Strictly, these form a distinct class of helix but they are always shortĪnd frequently occur at the termini of regular alpha-helices. This gives rise to a bend in the helix axis. This is because the exposed C=O groups tend to point towards solvent to maximise their H-bonding capacity, ie tend to form H-bonds to solvent as well as N-H groups. Exposed helices are often bent away from the solvent region. Proline occurs more commonly in extended regions of polypeptide. Helices containing proline are usually long perhaps because shorter helices would be destabilised by the presence of a proline residue too much. Janet Thornton has shown that proline causes two H-bonds in the helix to be broken since the NH group of the following residue is also prevented from forming a good hydrogen bond. This is because proline cannot form a regular alpha-helix due to steric hindranceĪrising from its cyclic side chain which also blocks the main chain N atom and chemically prevents it forming a hydrogen bond. Proline residues induce distortions of around 20 degrees in the direction of the helix axis.The packing of buried helices against other secondary structure elements in the core of the protein.
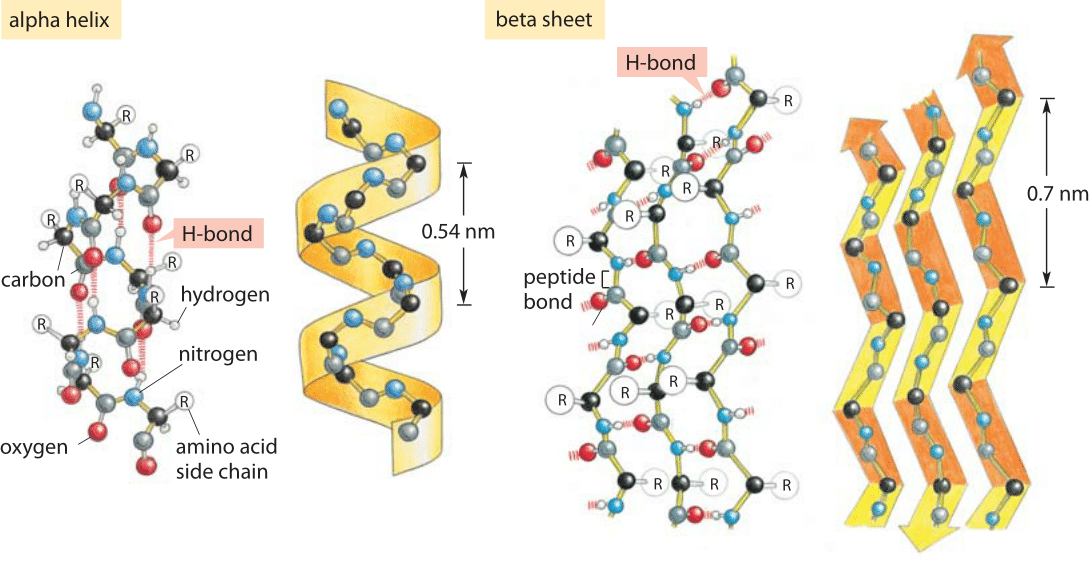
These distortions arise from several factors including: The majority of alpha-helices in globular proteins are curved or distorted somewhat compared with the standard Pauling-Corey model. All the amino acids have negative phi and psi angles, typical values being -60 degrees and -50 degrees, respectively.Side chains point outward from helix axis and are generally oriented towards its amino-terminal end. The peptide planes are roughly parallel with the helix axis and the dipoles within the helix are aligned, ie all C=O groups point in the same direction and all N-H groups point the other way.This gives a very regular, stable arrangement. Every mainchain C=O and N-H group is hydrogen-bonded to a peptide bond 4 residues away (ie O(i) to N(i+4)).The separation of residues along the helix axis is 5.4/3.6 or 1.5 Angstroms, ie the alpha-helix has a rise per residue of 1.5 Angstroms. Alpha-helices have 3.6 amino acid residues per turn, ie a helix 36 amino acids long would form 10 turns. The structure repeats itself every 5.4 Angstroms along the helix axis, ie we say that the alpha-helix has a pitch of 5.4 Angstroms.


 0 kommentar(er)
0 kommentar(er)
